The Application Form for Export of Narcotic Drugs is a important document that helps the legal export of controlled substances and ensures compliance with international regulations. This form serves as a standardized framework for exporters to apply for permission to export narcotic drugs for medical, scientific, or research purposes.
Exporting narcotic drugs requires meticulous documentation and adherence to strict guidelines to prevent misuse and maintain public safety. The application form acts as a complete tool to gather essential information, including details of the exporter, recipient, quantity of drugs, purpose of export, and relevant permits.
The Application Form for Export of Narcotic Drugs also promotes transparency and accountability in the export process. It allows authorities to track the movement of narcotic drugs, preventing unauthorized diversion or illicit trade.
Details for Application Form for Export of Narcotic Drugs and Psychotropic Substances
Exporter’s Information
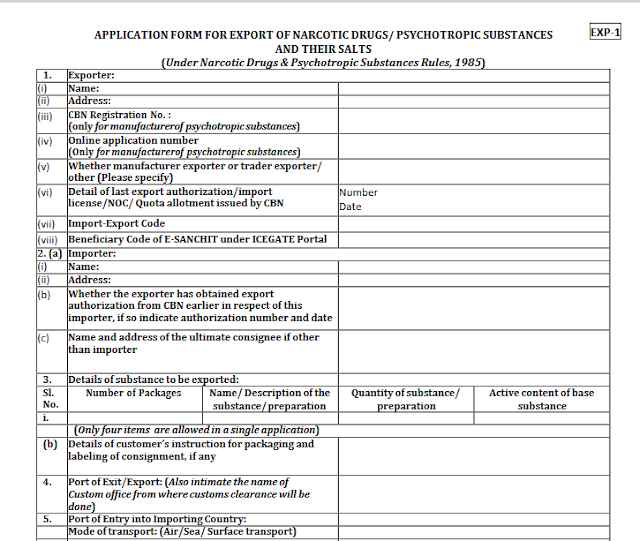
- Name
- Address
- CBN Registration No. : (only for manufacturerof psychotropic substances)
- Online application number (Only for manufacturerof psychotropic substances)
- Whether manufacturer exporter or trader exporter/ other (Please specify)
- Detail of last export authorization/import license/NOC/ Quota allotment issued by CBN
- Import-Export Code
- Beneficiary Code of E-SANCHIT under ICEGATE Portal
Importer’s Details
- Name
- Address
- Whether the exporter has obtained export authorization from CBN earlier in respect of this importer, if so indicate authorization number and date
- Name and address of the ultimate consignee if other than import
- Details of substance to be exported
- Details of customer’s instruction for packaging and labeling of consignment, if any
- Port of Exit/Export: (Also intimate the name of Custom office from where customs clearance will be done)
- Port of Entry into Importing Country
- Mode of transport: (Air/Sea/ Surface transport)
- Name of the transshipment country/port, if any
- Details of Import Certificate/ Permit or any relevant document issued by the Competent National Authority:
- No. & Date:
- Name of Issuing Authority
- Details of intermediaries in India and abroad (if any) involved in the transaction
- Whether export is meant for re-export, Specify country(ies){if known) and quantities {if known}
- Details of Drug License issued by the State Drugs Controller/ FDA:
- Details of State Excise permit (in case of Narcotic drugs):
- If you had obtained Quota Allocation of any Narcotic Drugs for the purpose of export & you wish that this export is to be logged against that Quota Allotment then indicate the number and date of Quota Allotment
- Details of fee for export authorization:
Details of documents required to be submitted along with the application
- Original copy of Import permit issued by CNA of importing country (wherever applicable). If the certificate/ permit is not in English language, an authentic copy translated in English shall have to be enclosed with the original copy
- Licence Fee of Rs. 1000/- in form of Demand Draft drawn in favor of Drawing and Disbursing Office, Central Bureau of Narcotics, Gwalior.
- Copy of valid drug manufacturing license possessed by the exporter (in case of manufacturer-exporter). To be submitted at the beginning of calendar year along with their first application and also immediately after the renewal of their drug license. In subsequent application only reference of previous application in which this document has been submitted is to be given.
- Copy of valid drug license for sale, distribution or exhibit (in case of trader- exporter) along with copy and relevant portion of approved product list of manufacturer of the substance to be exported. To be submitted at the beginning of calendar year along with their first application and also immediately after the renewal of their drug licence. In subsequent application only reference of previous application in which this document has been submitted is to be given.
- Copy of the purchase order placed by the foreign buyer/intermediaries/ agent for the proposed import.
- Attested copy of NOC or Additional Licence/Permission required under the provisions of Drug & Cosmetic Act/Rules, if applicable
- For Narcotic drugs- Original copy of excise permit in case of exporter who are dealing with CBN for less than 1 year and certified copy of excise permit for the exporter who have been dealing with CBN for more than 1 years.
List of additional Documents to be submitted by first time exporter/import of Narcotic Drugs, Psychotropic Substances and for existing Importer/Exporters dealing with CBN, once in January of each year or with first application of that year or whenever there is any change in details.
- Complete postal address and telephone, fax no. of various factories of the company manufacturing Narcotic Drugs and Psychotropic Substances including Jurisdictional GST division and GST Commissionerate and Zonal office of Narcotics Control Bureau in respect of factories.
- Name, address, telephone Nos. and Fax No. of the Chairman, Managing Director and other Directors, proprietor/ partners, in charge of production and finance. and / PAN of directors/proprietors/partners & DIN (For companies only)
- GST Registration No., Company’s PAN No. , IEC and CIN Number (for companies only)(Attested copies of these documents shall be submitted.)
- Turnover of the company for last three years in crore.
- Original Authority letter (in company's/ firm's letter head) for signatories signing on behalf of the company/ firm issued by the Board of Directors/ Partners/ Proprietors.
Application Form for Export of Narcotic Drugs/ Psychotropic Substances and Their Salts PDF DOWNLOAD
Also Download








0 Comments